Innovation drives progress. When it comes to innovation in the development of new drugs and therapeutic biological products, FDA ’s Center for Drug Evaluation and Research (CDER) supports the. New Approvals Report (PDF - MB) Text Version.

The products in each list contain information about what medical uses the device is cleared or. The increased approvals are consistent with FDA ’s policy to eliminate backlogs and expedite such orphan drug reviews. The FDA approved a record drugs last year, but the commercial potential of these drugs is lacklustre.
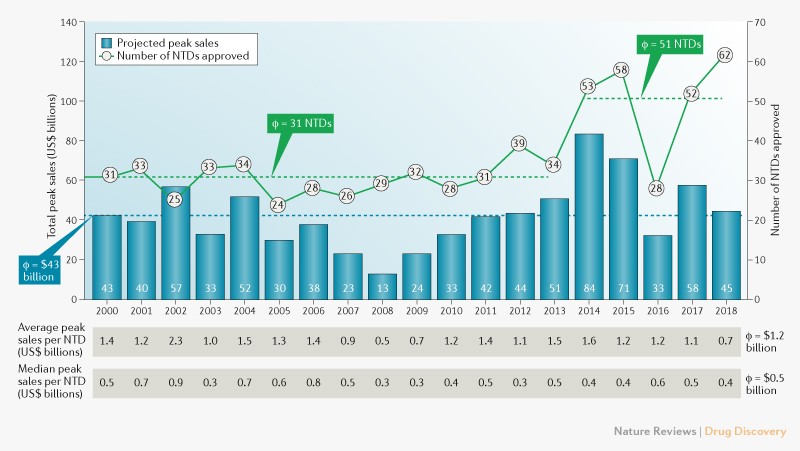
Asher Mullard Search for this author in:. Federal government websites often end in. Up to date information on the latest FDA drug approvals.
Includes list of most recent approvals , the conditions approved for, and the approval history. Die Wegbereiter für kluges Online-Shopping - jeder Kauf eine gute Entscheidung! Sicher durch den Winter. Bewegungsanalyse-Software, Einfache Anwendung in Medizin und Sport. Schau Dir Angebote von Fda V1k auf eBay an.
This year showed the highest number of new drug approvals in the last two decades. Previously, the vaccine was only approved up to age 26. For purpose of FDA review, the term NME applies to novel drugs approved under both New Drug Applications (NDAs) and Original Biologics License Applications (BLAs). Medical device manufacturers last year benefited from a higher number of novel medical device approvals than any other time in FDA history, joining branded and generic drug manufacturers who also saw record-setting approvals , according to the FDA. Our enhanced FDA calendar integrates PDUFA dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers all companies facing upcoming PDUFA dates.
Streamline your research and quickly compare the relative timing of competing catalysts. All supporting data can be copied to the clipboard. Biotech Stock Catalyst and FDA Calendar for your biotech stock investing. Use our tools on your road to profit in the stock market. Phase catalysts for small-cap companies only are listed.
Der AutoScoutMarktplatz hat auch Ihren DAF. Deutschlands größter Preisvergleich - mehrfacher Testsieger mit TÜV-Zertifikat! Paratek Pharmaceuticals, “Paratek Announces FDA Approval of NUZYRA (Omadacycline),” Press Release, Oct. Tetraphase Pharmaceuticals, “Tetraphase Pharmaceuticals Announces FDA Approval of XERAVA (Eravacycline) for Complicated Intra-Abdominal. Food and Drug Administration ( FDA ) announced three approvals of anticancer therapeutics.
FDA Commissioner Scott Gottlieb has made speeding up review of generics an agency priority, as a lever to help bring down drug prices. An Analysis of FDA Drug Approvals from the Perspective of Molecules Beatriz G. The US FDA ’s approval of Krintafel is a major milestone and a significant contribution towards global efforts to eradicate malaria,” commented David Reddy, Ph chief executive officer of MMV in a recent statement, “The world has waited decades for a new medicine to counter P vivax malaria relapse. Today, we can say the wait is over. An FDA review found that in such patients, spasm of the sphincter of Oddi may lead to severe pancreatitis.
The FDA reported that in some cases symptoms have occurred with just one or two doses at the recommended dosage for patients without a gallbladder (mg). Das Leben läuft nicht immer nach Plan. Flexibel bleiben mit dem Sofortkredit!
Setzt einen Meilenstein für die Zukunft im Banking - tagesspiegel.
Keine Kommentare:
Kommentar veröffentlichen
Hinweis: Nur ein Mitglied dieses Blogs kann Kommentare posten.