Innovation drives progress. Federal government websites often end in. An essential round-up of science news, opinion and analysis, delivered to your inbox every weekday. The following drugs have recently been approved by the FDA. Includes newly approved drugs and new indications for drugs already approved.
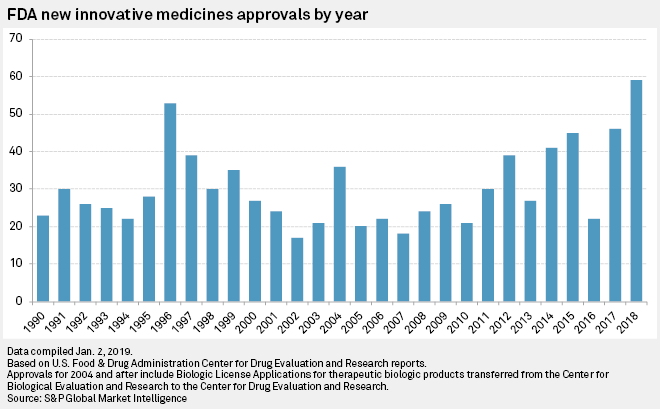
Many accelerated drug approvals. It takes on average years and over US$3million to get a new drug from the laboratory onto the pharmacy shelf. Once a company develops a drug , it undergoes around three and a half years of laboratory testing, before an application is made. The list also contains drugs that are first in class and 43.
Based on information in FDA and company press releases, approximately half of the new drugs were approved under an expedited review process—Fast Track, Breakthrough Therapy, Priority Review, Accelerated Review—or orphan drug status. The last phase of drug approval is an ongoing one while the drug is on the marketplace. If a developer wants to change anything about the drug formulation or approve it for a new use, they must apply with the FDA. The FDA also frequently reviews the drug ’s advertising and its manufacturing facility to make sure everything involved in its. Here are the stories that made headlines on cancer.
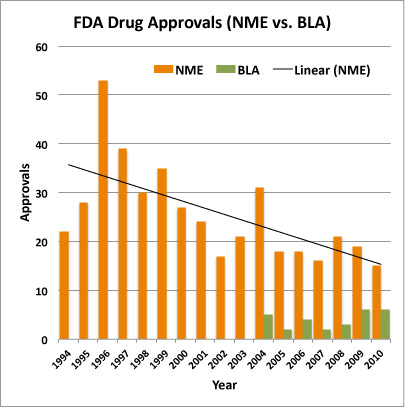
Altogether, there were drugs with at least one divergent regulatory outcome at the EMA and the FDA among the three types examined: drugs had different initial decisions on marketing approval an after accounting for resubmission or reexamination of certain applications, drugs had a different final decision on marketing approval. Novel drug approvals also increased in Europe. The FDA must approve new drug compounds before drug companies can market or sell the drug in the U. However, the FDA isn’t responsible for developing drugs , nor does the organization conduct any testing. In reality, the only role the FDA has in the testing of drugs is reviewing data drug sponsors gather and submit.
Still, FDA approval of esketamine for treating depression comes with plenty of caveats. Drug Approval Process. The FDA continues to support the development of new drug therapies for diabetes management,” said Mary Thanh Hai Parks, M. New FDA Approval Report. Adlyxin will add to the available treatment options to control blood sugar levels for those with type 2. S Government Accountability.
First, the company must conduct laboratory tests and try the drug on animals and then people to make sure it works and is safe. Pretomani developed by the non-profit TB Alliance, has received U. Officially, there were new molecular. Should the FDA speed up or slow down approval of new cancer drugs ? It’s been an exciting year for new drug approvals ! This is up from last year’s.
FDA ’s approval of new TB drug comes amid alarm over rise of drug -resistant forms of an ancient infectious disease. Pretomanid would be taken with two other pills to treat highly treatment. Four Rules For Being A Consistent US FDA Reviewer From Peter Stein Pink Sheet. An expert panel with the FDA voted for the approval of a new treatment option for children with peanut allergies. The treatment would be the first drug approved to prevent, or at least mitigate.
It is one a kind drug. Moreover, it also has a fixed regime. Therefore it is the first of this kind to get approvals of FDA. The approval of this drug comes as progress.
FDA approves new vaginal ring for one year of birth control The U. The drug , therefore, will give HIV patients a better life.
Keine Kommentare:
Kommentar veröffentlichen
Hinweis: Nur ein Mitglied dieses Blogs kann Kommentare posten.